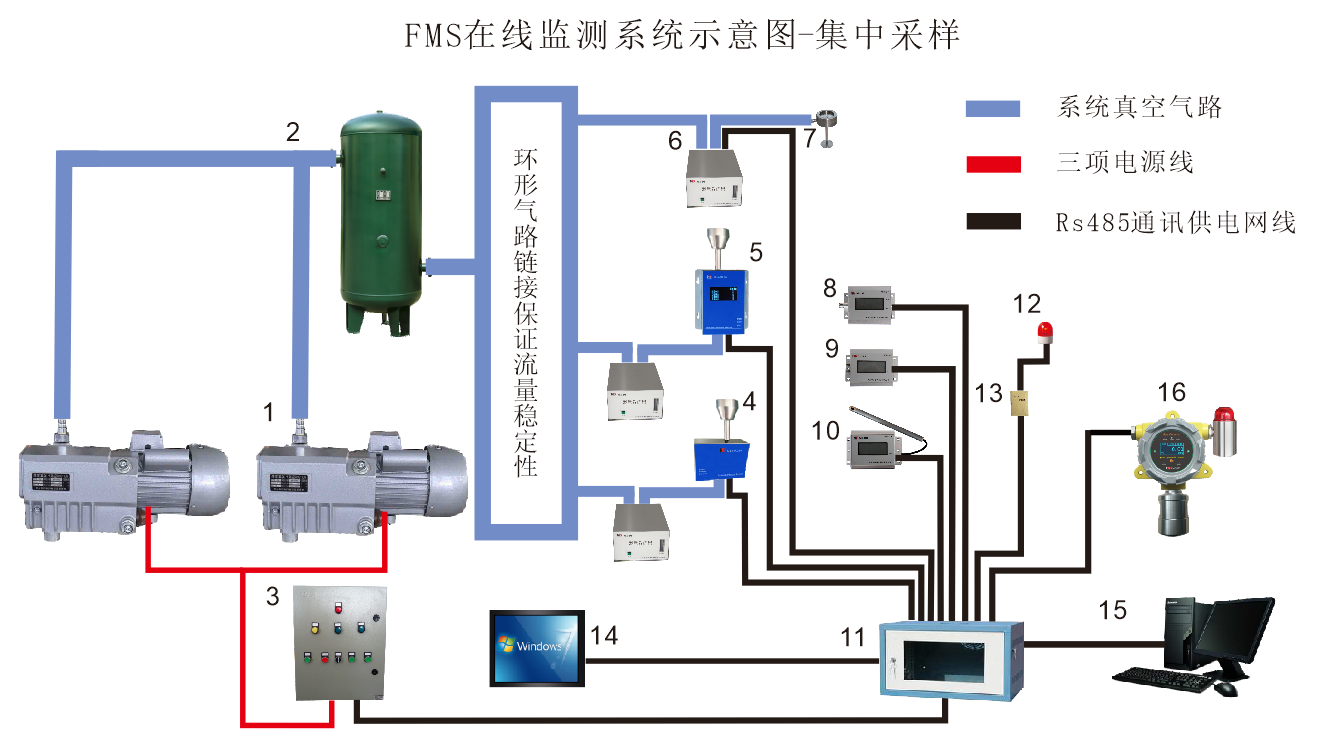
The HR-FMS by Suzhou Honri Purification Technology is an integrated compliance solution designed specifically for the pharmaceutical industry. It adheres to international authoritative standards, including EU GMP Annex 1 (2022), US FDA 21 CFR Part 11, and ISO 14644-1:2015. The system provides 24/7 continuous monitoring of airborne particles, microorganisms, and environmental parameters to ensure data integrity and full lifecycle traceability.
II. Core Compliance Principles
-
Continuous Control: Replaces manual inspections with automated 24h data collection to eliminate the risk of unrecorded deviations.
-
Data Integrity: Fully compliant with 21 CFR Part 11 for electronic records and signatures, ensuring audit trails are non-tamperable.
-
Scientific Sampling: Adheres to ISO 14644-1 ($N=\sqrt{A}$) for point calculation and ensures isokinetic sampling in Grade A zones to maintain laminar flow.
III. Hardware Specifications
| Parameter | Model | Key Specifications | Compliance |
|---|---|---|---|
| Airborne Particles | CLJ-R310/R310P | 28.3L/min; Monitors 0.3/0.5/5.0 μm particles. | EU GMP Annex 1 & ISO 14644-1. |
| Active Air Sampling | FSC-R10 | Collection efficiency ≥98%; Remote real-time sampling. | Meets <1 cfu limit for Grade A. |
| Differential Pressure | DP-30R/DP-25 | Range: 0-±125 Pa; Response time <3s. | Prevents backflow and contamination. |
| Air Velocity | WS-30R/WS-25 | Accuracy: ±0.1 m/s; Monitors 0.36-0.54 m/s flow. | ISO 14644-4 airflow design. |
| Temp & Humidity | TH-30R/TH-25 | Temp: ±0.3℃; Humidity: ±3%RH. | Meets international IAQ standards. |
IV. Software Capabilities
-
Audit Trail: Features three-level permission management (Admin, Engineer, Operator) for total accountability per 21 CFR Part 11.
-
Smart Alarms: Multi-level thresholds with alerts via on-site sirens, SMS, and the Movicom mobile APP.
-
Compliance Reporting

Reviews
There are no reviews yet.